#cancer signaling pathways
Explore tagged Tumblr posts
Text
youtube
#Proteogenomics#Lung cancer#Cancer research#Oncology#Precision medicine#Biomarkers#Genomics#Proteomics#Molecular oncology#Tumor profiling#Personalized medicine#Targeted therapies#Cancer diagnostics#Clinical investigation#Cancer genetics#Translational research#Protein expression#Cancer signaling pathways#Therapeutic targets#Patient outcomes.#Youtube
0 notes
Text
TRPS1 as possible unique biomarker for triple-negative? Investigations on cellular biology and mechanisms
The mammary gland is a complex tissue comprising a variety of cell types. Their proper functioning is crucial for the health of the breast. Breast cancer is the widest condition affecting breast health and can be categorized as estrogen receptor (ER)/progesterone receptor (PR)-positive luminal, human epidermal growth factor receptor 2 (HER2)-positive, or triple-negative breast cancer (TNBC) types…

View On WordPress
#breast cancer#cell proliferation#cellular signaling#estrogen receptor#luminal progenitor cells#signaling pathway#transcription factor#triple-negative
0 notes
Text
Drug resistance in multiple myeloma: When cancer cells say "NO" to treatment
Drug resistance is like a game of cat and mouse. Cancer cells are the cat, and researchers are the mouse. The cat is always trying to find new ways to catch the mouse, but the mouse is always trying to find new ways to avoid getting caught.
Read More
#multiple myeloma#molecular mechanisms#signaling pathways#Health#stem cell transplantation#oncogenes#Lifestyle#cancer cells#gene mutations#drug resistance#cellular environment#gene expression
0 notes
Text
Submitted via Google Form:
How reckless could a race of people be if they could heal rapidly? If someone got hit by a car in real life and needed a few months to start walking again, and over a year to do anything like martial arts again, for these people, they'd be walking in a few days and doing martial arts in two weeks. If they would die on impact, then yes, they would die. If they would die in an ambulance, it depends but they would still have a chance. In real life, cuts that take a few days to heal will be healed in several minutes. They definitely do feel pain of course, but as a whole, the general populace has pain tolerance just below those of elite athletes in the real world. I'm imagining these people might be the daredevil type.. could it be very common to get cuts and bruises, not strange to see some of the worst kids or teens getting cut nearly every single day, multiple times a day. I mostly want to focus on the injured = not a big deal bit. Or getting injured as part of normal daily life. But what else might this affect? More surgeries happening because people decide the recovery time isn't an issue (doesn't eliminate other factors of course) How about piercings? If someone goes half a day without piercings, their hole would close. Maybe... medicine that stops the healing process? But how expensive could those medicines be vs getting pierced again?
Tex: So there’s this plant, called plantain. One of the common varieties is known as Plantago major. One of its primary uses in herbal medicine is to heal wounds - and it’s very good at it!
A little bit too good, unfortunately, and it often comes with a warning to clean out wounds first before applying it, because it has the tendency to work so quickly at encouraging skin to knit back up that infections can easily be sealed underneath. This is a problem, because in order to heal the infection, the skin must be cut back open, lest someone risk the infection spreading to the blood and causing sepsis (if not, in bad cases, necrosis).
“Super healing” has many of the same flaws. In practice, the process of healing is rather complex, and while there is some overlap in steps (excess blood cleared away, immune system response to pathogens, phagocytosis, signals sent to regenerate broken tissue or other affected organs), doing too much of only one process can have detrimental effects on the patient in question. It’s the reason why in first aid you clean a wound first, then apply medicines, then apply bandages.
Things like bones, and the squishier bits called organs, take time to heal, because they’re not only reallocating resources to grow new cells (i.e. neurogenesis, osteogenesis, etc), they’re also going through the entire pathway of fighting infections (i.e. B cells, T cells, etc) and checking for cancerous markers of cells that duplicated incorrectly (uncommon, but non-zero possibility). It’s a lot, lot more than “add calcium to bone” or “make skin whole”.
Regeneration of tissue is also rather itchy, and uncomfortable. That, barring anything else, is going to make a lot of people think twice about how many injuries they’re willing to risk. Compounding injuries compounds the discomfort, and most people wish to avoid being uncomfortable if there’s any other option for a situation.
On top of that, rapid regeneration would require a large amount of resources for both calories and micronutrients. This translates to being hungry all the time. Humans can generally heal quickly with a good diet and enough sleep (the brain regulates the flushing of metabolic waste during sleep, Patel et al.), which is why it’s seen as a good sign for hospital patients to have an appetite and also to have a regular sleep schedule.
You can handwave as much of this as you like in your worldbuilding, but to borrow SAW’s general rule, “you break it, you bought it” in terms of internal consistency.

23 notes
·
View notes
Text
Reference saved in our archive
Covid's making it difficult for people's immune systems to suppress cancer. There was a recent small-scale mouse study showing "tumor shrinkage" in a small percentage of those transgenic mice. This is a much larger analysis of the oncogenic potential of covid in humans. Guess which story the news has been reporting.
Abstract The 2019 outbreak of SARS-CoV-2 has caused a major worldwide health crisis with high rates of morbidity and death. Interestingly, it has also been linked to cancer, which begs the issue of whether it plays a role in carcinogenesis. Recent studies have revealed various mechanisms by which SARS-CoV-2 can influence oncogenic pathways, potentially promoting cancer development. The virus encodes several proteins that alter key signaling pathways associated with cancer hallmarks. Unlike classical oncogenic viruses, which transform cells through viral oncogenes or by activating host oncogenes, SARS-CoV-2 appears to promote tumorigenesis by inhibiting tumor suppressor genes and pathways while activating survival, proliferation, and inflammation-associated signaling cascades. Bioinformatic analyses and experimental studies have identified numerous interactions between SARS-CoV-2 proteins and cellular components involved in cancer-related processes. This review explores the intricate relationship between SARS-CoV-2 infection and cancer, focusing on the regulation of key hallmarks driving initiation, promotion and progression of cancer by viral proteins. By elucidating the underlying mechanisms driving cellular transformation, the potential of SARS-CoV-2 as an oncovirus is highlighted. Comprehending these interplays is essential to enhance our understanding of COVID-19 and cancer biology and further formulating strategies to alleviate SARS-CoV-2 influence on cancer consequences.
#mask up#wear a mask#public health#pandemic#wear a respirator#covid 19#covid#still coviding#coronavirus#sars cov 2#cancer
19 notes
·
View notes
Text
LT-α [TNF-beta] plays an important role in innate immune regulation and its presence has been shown to prevent tumor growth and destroy cancerous cell lines.[14] In contrast, unregulated expression of LT-α can result in a constantly active signaling pathway, thus leading to uncontrolled cellular growth and creation of tumors.[13]
The Janus faces of these proteins
8 notes
·
View notes
Text
This scoping review highlights the rapidly emerging threat of microplastic contamination within the human urinary tract, challenging the World Health Organization's assertion that microplastics pose no risk to public health. The documented cytotoxic effects of microplastics, alongside their ability to induce inflammation, reduce cell viability and disrupt signalling pathways, raise significant public health concerns relating to bladder cancer, chronic kidney disease, chronic urinary tract infections and incontinence. As a result, this study emphasises the pressing need for further research and policy development to address the challenges surrounding microplastic contamination.
Bond University research study
2 notes
·
View notes
Text
Unlocking The Potential Of Lenvatinib A Comprehensive Guide Understanding Lenvatinib Price
In the field of cancer treatment, where targeted medicines are changing the landscape of care, Lenvatinib 10 mg stands out as a promising medication for patients suffering from certain types of cancer; however, the lenvatinib price fluctuatesoccasionally. This article will provide a thorough examination of Lenvatinib 10 mg, providing light on its applications, mechanism of action, dosage, ability, side effects, and revolutionary effect on cancer treatment.
Introduction to Lenvatinib 10 mg
Lenvatinib is a tyrosine kinase inhibitor with demonstrated success in the treatment of a variety of malignancies, including thyroid cancer and hepatocellular carcinoma (HCC). The 10 mg dosage of Lenvatinib is a specific method geared to meet the healing needs of patients undergoing targeted treatment.
Mechanism of Action
Lenvatinib works by inhibiting a few receptor tyrosine kinases (RTKs), as well as vascular endothelial growth problem receptors (VEGFRs), fibroblast increase detail receptors (FGFRs), and platelet-derived boom element receptors (PDGFRs). Lenvatinib exerts anti-cancer effects by focusing on the major signaling pathways involved in tumor angiogenesis, development, and metastasis, which are ultimately necessary for tumor regression and advanced impacted character outcomes.
Uses of Lenvatinib 10 mg
Thyroid Cancer
Lenvatinib 10 mg is approved for the treatment of differentiated thyroid cancer (DTC) that is resistant to radioactive iodine therapy. It has demonstrated efficacy in slowing disease progression and improving improvement-free survival in patients with advanced or metastatic DTC. You can purchase it by obtaining information on lenvatinib prices from medical clinics or online.
Hepatocellular Carcinoma (HCC)
For patients with advanced HCC who are not candidates for surgical resection or network ablation, Lenvatinib 10 mg provides a valuable therapy option. It has demonstrated superiority over sorafenib, an excellent tyrosine kinase inhibitor, in terms of overall survival and progression-free survival in patients with unresectable HCC.
Dosages and Administration
Lenvatinib 10 mg is typically delivered orally once per day, with or without food. The dosage can be changed based on the affected person’s characteristics, such as frame weight, renal function, and tolerability. Healthcare personnel regularly monitor patients getting Lenvatinib medication, including regular examinations of tumor reactions and adverse effects to optimize treatment outcomes.
Potential Side Effects
While Lenvatinib 10 mg is generally well tolerated, it may produce adverse outcomes in certain patients. Common side effects of Lenvatinib medication include elevated blood pressure, lethargy, diarrhea, decreased appetite, nausea, and proteinuria. Patients are advised to immediately report any new or worsening symptoms to their healthcare providers for proper management.
Wrapping Up
Finally, Lenvatinib 10 mg represents a significant development in the treatment of thyroid cancer and hepatocellular carcinoma, providing improved outcomes and increased survival for patients with advanced or metastatic disease. Its concentrated mechanism of action, combined with its broad range of medical warning signals, emphasizes its importance in modern oncology. Patients are recommended to consult with their healthcare providers to learn more about the lenvatinib price, for personalized guidance, and for adapted control strategies when using Lenvatinib 10 mg. People can begin their treatment path with confidence and hope for a better future by understanding the intricacies of most cancers and investigating available therapy alternatives. Source:-
Unlocking The Potential Of Lenvatinib A Comprehensive Guide Understanding Lenvatinib Price
2 notes
·
View notes
Text
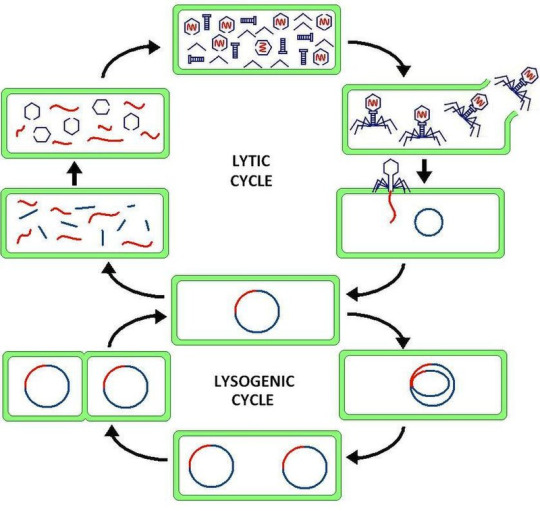
Virus latency (or viral latency) is the ability of a pathogenic virus to lie dormant (latent) within a cell, denoted as the lysogenic part of the viral life cycle.[1] A latent viral infection is a type of persistent viral infection which is distinguished from a chronic viral infection. Latency is the phase in certain viruses' life cycles in which, after initial infection, proliferation of virus particles ceases. However, the viral genome is not eradicated. The virus can reactivate and begin producing large amounts of viral progeny (the lytic part of the viral life cycle) without the host becoming reinfected by new outside virus, and stays within the host indefinitely.[2]
Episomal latency refers to the use of genetic episomes during latency. In this latency type, viral genes are stabilized, floating in the cytoplasm or nucleus as distinct objects, either as linear or lariat structures. Episomal latency is more vulnerable to ribozymes or host foreign gene degradation than proviral latency (see below).
Advantages of episomal latency include the fact that the virus may not need to enter the cell nucleus, and hence may avoid nuclear domain 10 (ND10) from activating interferon via that pathway. Disadvantages include more exposure to cellular defenses, leading to possible degradation of viral gene via cellular enzymes.[12]
Proviral latency: A provirus is a virus genome that is integrated into the DNA of a host cell
All interferons share several common effects: they are antiviral agents and they modulate functions of the immune system. Administration of Type I IFN has been shown experimentally to inhibit tumor growth in animals, but the beneficial action in human tumors has not been widely documented. A virus-infected cell releases viral particles that can infect nearby cells. However, the infected cell can protect neighboring cells against a potential infection of the virus by releasing interferons. In response to interferon, cells produce large amounts of an enzyme known as protein kinase R (PKR). This enzyme phosphorylates a protein known as eIF-2 in response to new viral infections; the phosphorylated eIF-2 forms an inactive complex with another protein, called eIF2B, to reduce protein synthesis within the cell. Another cellular enzyme, RNAse L—also induced by interferon action—destroys RNA within the cells to further reduce protein synthesis of both viral and host genes. Inhibited protein synthesis impairs both virus replication and infected host cells. In addition, interferons induce production of hundreds of other proteins—known collectively as interferon-stimulated genes (ISGs)—that have roles in combating viruses and other actions produced by interferon.[13][14] They also limit viral spread by increasing p53 activity, which kills virus-infected cells by promoting apoptosis.[15][16] The effect of IFN on p53 is also linked to its protective role against certain cancers.[15]
Another function of interferons is to up-regulate major histocompatibility complex molecules, MHC I and MHC II, and increase immunoproteasome activity. All interferons significantly enhance the presentation of MHC I dependent antigens. Interferon gamma (IFN-gamma) also significantly stimulates the MHC II-dependent presentation of antigens. Higher MHC I expression increases presentation of viral and abnormal peptides from cancer cells to cytotoxic T cells, while the immunoproteasome processes these peptides for loading onto the MHC I molecule, thereby increasing the recognition and killing of infected or malignant cells. Higher MHC II expression increases presentation of these peptides to helper T cells; these cells release cytokines (such as more interferons and interleukins, among others) that signal to and co-ordinate the activity of other immune cells.[17][18][19]
Epstein–Barr virus lytic reactivation (which can be due to chemotherapy or radiation) can result in genome instability and cancer.[5]
HSV reactivates upon even minor chromatin loosening with stress,[7] although the chromatin compacts (becomes latent) upon oxygen and nutrient deprivation.[8]
Cytomegalovirus (CMV) establishes latency in myeloid progenitor cells, and is reactivated by inflammation.[9] Immunosuppression and critical illness (sepsis in particular) often results in CMV reactivation.[10] CMV reactivation is commonly seen in patients with severe colitis.[11]
viral latency is so fucked up
11 notes
·
View notes
Text
literally every time i go to cancer bio i pay attention for like 20 minutes and then just start zoning out without fail it's kind of uncanny the ability this class has to make me just fall asleep. it's really good i decided to not go to medical school because i find this really like granular discussion of pathways fucking intolerable to be honest like i do not gaf about individual signaling cascades i care so much more about the gross effects and systems as a whole. if i had to memorize 1000 hormones and signaling proteins and whatever i would probably turn into the joker like genuinely.
2 notes
·
View notes
Text

Targeted Therapy:
Precision or targeted therapies encompass medications engineered to disrupt specific molecules implicated in the progression of cancer. In contrast to conventional chemotherapy's broad impact on fast-dividing cells, precision therapies selectively target cancer cells while preserving healthy tissue integrity. These drugs aim at various molecular pathways involved in cancer development, including signaling cascades, angiogenesis, and DNA repair mechanisms.
An illustrative example of precision therapy is the application of tyrosine kinase inhibitors (TKIs) in treating specific cancer types like non-small cell lung cancer (NSCLC) and chronic myeloid leukemia (CML). TKIs hinder the activity of particular tyrosine kinases, crucial enzymes in cancer-promoting cell signaling pathways. By obstructing these kinases, TKIs effectively inhibit tumor growth and extend patient survival.
Likewise, monoclonal antibodies represent another form of precision therapy, binding to specific proteins on cancer cell surfaces, initiating immune-mediated tumor destruction. These antibodies can also be combined with cytotoxic agents or radioactive isotopes to heighten their anti-cancer properties.
Personalized Chemotherapy:
While precision therapies are central to personalized medicine, tailored chemotherapy remains vital in cancer treatment. Tailored chemotherapy involves customizing traditional cytotoxic drugs to suit the unique characteristics of each patient's tumor. This may involve adjusting drug doses, combining different agents, or selecting chemotherapy regimens based on tumor biology and patient-specific factors.
One approach to tailored chemotherapy utilizes predictive biomarkers to identify patients likely to respond positively to specific chemotherapy drugs. For example, certain mutations in the BRCA genes are associated with increased sensitivity to platinum-based chemotherapy in breast and ovarian cancers. By identifying these biomarkers, oncologists can identify patients who will benefit most from a particular chemotherapy regimen while minimizing potential toxicity for others.
Furthermore, progress in pharmacogenomics, which explores how genetic variations affect drug response, has provided insights into individual differences in drug metabolism and toxicity. By analyzing patients' genetic profiles, oncologists can predict their likelihood of experiencing adverse effects or poor response to chemotherapy drugs, enabling personalized dose adjustments and treatment optimization.
Early cancer detection and management is important for an improved success rate in cancer treatment. You can undergo regular health checkups to get diagnosed for cancer at an early-stage. There are many good hospitals in Mumbai that offer health checkup packages for cancer screening, such as a full body health checkup at Saifee Hospital Mumbai, which is one of the best hospitals in the country.
#chemotherapy#personalized chemotherapy#targeted therapy#full body health checkup#regular health checkups#cancer screening#cancer detection
4 notes
·
View notes
Text
youtube
#Hepatocellular carcinoma#LRP1 loss#UFL1#NF-κB signaling#liver cancer progression#tumor proliferation#cell migration#apoptosis resistance#cancer signaling pathways#oncogenesis#inflammation in cancer#tumor microenvironment#cancer therapy targets#molecular oncology#cell survival mechanisms#HCC progression#therapeutic strategies#cancer suppression#oncogenic signaling#liver tumor biology.#Youtube
0 notes
Note
🧪 (this is such a fun idea!)
So in my lab, we study cells in biofilms. Biofilms are surface-adhered aggregates of microbes living in a matrix of biomolecules that they secrete. The really cool things is that even though a biofilm is a community of individual microbes, not an organism, it actually takes on a lot of multicellular properties. The current thinking is that multicellularity might have arisen as a result of cells living together in biofilms.
The specialization that occurs within biofilms is analogous to differentiation. Biofilms are heterogeneous, even when they are comprised of a clonal population (in which all the cells are genetically identical). There's a division of labor that occurs as a result of stochastic gene expression: one subpopulation of cells might only excrete exopolysaccharides, for example, while another works exclusively at nucleic acid metabolism. This division of labor isn't a result of environmental factors; it's a result of communication between the cells in the biofilm.
But this goes even further - in biofilms, cooperation is actually prioritized over competition between cells, which for a long time was hard to explain from an evolutionary standpoint. Biofilms commonly have cannibalism pathways: as the biofilm matures, the community signals cell types that are no longer needed to lyse -- and they do! After that, the remaining cells - especially the types that are most useful/energetically expensive to the community - will metabolize the remains of the lysed cells. This is darn similar to apoptosis, or pre-programmed cell death, in multicellular organisms. Apoptosis is the process that forms a baby's fingers and eyelids - the tissue tells particular cells to lyse so that gaps in the tissue can be created, then take in the nutrients they leave behind. It's also how pre-cancerous cells are frequently kept in check - the surrounding cells signal cells behaving abnormally to lyse.
Biofilms! Actually fit a number of the traditional "definition by description" criteria for living organisms as a unit rather than as individual cells! Biofilms adapt to their environments, grow and develop, and respond to stimulus. They evolve, after a fashion. Cell-to-cell communication allows biofilms to perform group behaviors all at once in a coordinated way, much as a multicellular tissue would.
This isn't to say that biofilms ought to be considered living organisms; clearly, they are composed of individual, typically prokaryotic microbes. But current thinking is that this is where the multicellular compact (cooperate, don't compete) began! Communities like this are very likely the predecessor to organisms in which millions of cells communicate and differentiate and cooperate. It's a really good transitional state, which is why I love studying communities like this.
If you happen to have institutional access, here is a really great paper on the subject.
Why does this point me to God? Because God gave us these crazy little communities that swap DNA back and forth and talk to each other and die for each other and then he gave us bodies that behave the same way! Just like the Bible is full of repeated ideas and motifs, just like good literature returns and returns to sets of themes, just like music does variations on a line or a theme or a chorus, so too with nature. The study of evolutionary biology is, at least in some respects, digging deep into what those themes are and using them to draw conclusions. Everything is everything; my body's cells and the cells in my petri dishes are more similar than I might ever have guessed.
#my research!#my PI can talk about biofilms being analogous to multicellular organisms for actual hours#it's awesome#ask me hard questions#all truth is god's truth#endless forms most beautiful
8 notes
·
View notes
Text
The Promising Future of Biochemistry Research: Unveiling the Mysteries of Life
Biochemistry, the scientific study of the chemical processes and substances that occur within living organisms, stands at the precipice of a remarkable era of discovery and innovation. As technology continues to advance and our understanding of molecular biology deepens, the future of biochemistry research holds the potential to revolutionize medicine, agriculture, energy production, and environmental sustainability.
Precision Medicine and Personalized Therapies: Biochemistry research is ushering in an era of personalized medicine, where treatments are tailored to an individual's unique genetic makeup and biochemical profile. The elucidation of intricate molecular pathways, protein structures, and genetic mutations empowers researchers to develop targeted therapies for diseases like cancer, neurodegenerative disorders, and rare genetic conditions. Precision medicine promises more effective treatments with fewer side effects, bringing hope to patients worldwide.
Synthetic Biology and Biotechnology: The fusion of biochemistry with engineering has given rise to synthetic biology and biotechnology. Researchers are designing novel biological systems, enzymes, and pathways for various applications, such as biofuel production, bioremediation, and drug synthesis. This field holds the potential to address pressing global challenges, including sustainable resource utilization and environmental restoration.
Structural Biology and Drug Discovery: Advances in biochemistry techniques, such as cryo-electron microscopy and X-ray crystallography, have revolutionized our ability to visualize the three-dimensional structures of biomolecules. This knowledge is invaluable for rational drug design, enabling scientists to develop new therapeutic agents that precisely target disease-causing molecules. The future holds the promise of faster and more accurate drug discovery, leading to improved treatment options for a wide range of ailments.
Neurobiochemistry and Brain Health: Exploring the intricate biochemical processes underlying brain function is shedding light on neurological disorders and paving the way for potential interventions. As our understanding of neurotransmitters, signaling pathways, and neuroplasticity deepens, biochemistry research could unlock innovative therapies for conditions such as Alzheimer's disease, Parkinson's disease, and mood disorders.
Biochemistry in Agriculture: Biochemistry is playing a pivotal role in enhancing crop yields, developing disease-resistant plants, and optimizing nutrient utilization in agriculture. By unraveling the molecular mechanisms governing plant growth, stress responses, and interactions with microorganisms, researchers are contributing to global food security and sustainable farming practices.
The future of biochemistry research is undeniably exciting, as it holds the key to transformative breakthroughs across a multitude of sectors. From personalized medicine to sustainable biotechnology and beyond, our growing knowledge of biochemical processes promises to reshape the way we address challenges and improve the quality of life for people around the world. As technology and collaboration continue to drive innovation in this field, the mysteries of life are gradually being unraveled, paving the way for a brighter and more promising future.
#science#biochemistry#research scientist#research paper#research laboratory#education#learn#learning#research chemicals#infographic#research newswire#scifi#scifiedit#scifiart
3 notes
·
View notes
Text
What is Cancer, Actually?

Ever since covid there has obviously been a huge spike in people distrusting the medical community- with everything. I've started to hear the argument, "oh, they don't want to cure cancer because they make so much money treating it!", which is stupid, but it's a lot easier to refute when you actually know what happens in your body when you have cancer. So what is cancer, actually?
In short, cancer is what happens when some of your cells mutate in a way that makes them grow uncontrollably. Normally, your cells receive signals telling them to grow and divide. The more times they divide, the greater the chance that they'll pick up mutations along the way. If the cells get too old or too damaged, however, they are equipped with a "kill switch" that triggers apoptosis, or programmed cell death. This does a pretty good job at removing damaged cells from your body. However, sometimes a cell will mutate in a way that it keeps growing regardless of what the signals are telling it, and loses its ability for apoptosis. When this happens, the cells will divide rapidly to form lumps of tissue called tumors. These can be benign in some cases, but in others they can be deadly. All that the cancer cells in the tumor care about is multiplying, no matter the cost. They may destroy healthy cells that get in their way, block the supply of nutrients or oxygen, or allow waste products to build up. They may even spread to other parts of the body, where they can continue to cause damage.
So if we know how cancer works, why haven't we cured it yet?
Cancer is not a single disease- it's an umbrella term for over 200 different conditions. And even though they all stem from the uncontrolled growth of cells, they are incredibly diverse, and often respond differently to different sorts of treatment.
In order to get rid of the cancer we need to kill the cancer cells. But since the cancer cells are just normal cells that got mutated, most ways of doing so will also damage the rest of your cells. Chemotherapy often works because it kills the fast-dividing cancer cells faster than it kills the rest of you (side note: common chemo side effects such as hair loss and nausea are due to the fact that the cells that your hair grows from and the cells of your stomach lining are also very fast-dividing, meaning that they often get harmed by the radiation as well). This doesn't work for all cancers, however, and for those we would need a way to kill the cancer cells that is either less harmful to the normal cells or targets only the cancerous cells. But that is very difficult to do, because...
There is not one single mutation that turns a normal cell into a cancer cell. the pathways that a cell can take into becoming cancer are endless, so treatments that work for cancer cells with a certain set of mutation might be entirely ineffective for others.
Because cancer cells divide so quickly and don't undergo apoptosis when they are damaged, the rate at which they can pick up new mutations dramatically increases. This means that they can change in a number of unpredictable ways, such as becoming resistant to treatments or learning to survive in different areas of the body.
So while these factors make it unlikely that cancer will be "cured" in the near future, people are constantly researching to discover new and more effective methods of treatment such as CAR T therapies. No doubt we will continue to see more and more progress as time goes on.
2 notes
·
View notes
Text
Does AA Work? New Open To Debate Podcast Episode For Dry January

Last week, the U.S. Surgeon General issued a sobering report about the cancer risks linked to something that most Americans enjoy frequently: an alcoholic beverage.
In the advisory, Dr. Vivek Murthy outlined the substantial evidence behind the increased risk of developing seven types of cancers among people who consumed as little as one daily drink, or even fewer.
"What we know with a high degree of confidence is that there is a causal link between alcohol and cancer risk," says Murthy as reported by Time Magazine. "The data has been building for some time and getting stronger and stronger."
The advisory cites alcohol as the third leading preventable cause of cancer in the U.S. after tobacco and obesity and notes that there are about 20,000 alcohol-related cancer deaths in the country annually. That's more than the yearly number of alcohol-associated traffic crash fatalities.
With that development in mind, the Open to Debate podcast has again crafted an episode that is timelyand critical.
Last year, a quarter of all Americans participated in Dry January -- it’s a popular campaign -- where one voluntarily abstains from alcohol for the entire month.
One outcome of Dry January for many — quitting alcohol entirely — is at the heart of this week’s episode of Open to Debate where the show discusses the group Alcoholics Anonymous (AA), which promotes a recovery process based on personal accountability, spiritual growth, and abstinence from alcohol while following the 12-step path.
But with 28.9 million people suffering from Alcohol Use Disorder in the past year, what are the pros and cons of different pathways for support? Supporters of Alcoholics Anonymous point to the millions of people worldwide who say AA saved their lives and credit its peer-driven, community-based approach. Those who think there might be better ways than the zero-sum approach of AA say it doesn’t work for everyone and that addiction often can require clinical interventions, such as therapy and medication.
Two experts on alcohol addiction and recovery debate whether AA works this week. Arguing “yes” is Dan Griffin, a speaker and author of “A Man’s Way Through the 12 Steps.” Arguing “no” is Adi Jaffe , the founder of the IGNTD recovery program and the author of the forthcoming book “Unhooked: Freeing Yourself From Addiction Forever.” Nayeema Raza, journalist and co-host of the Semafor Podcast “Mixed Signals”, guest moderates.
Open to Debate is a call to action: All of us should keep an open mind to solve the complex problems we face as individuals and as a nation.
Open to Debate is a reminder: To solve our greatest problems, we must operate in a contempt-free zone. We need to be able to sit in the same room and exchange ideas with people we disagree with. Being open to debate is a gesture of respect for the good faith arguments of those we disagree with, for the intelligence and integrity of those who watch or listen, and for the value of debate done right.
Does AA work? Listen to the debate now.
0 notes